- Immunizations

Immunizations are one of the best ways to prevent diseases and keep your family healthy. At the Benzie-Leelanau District Health Department, we offer routine vaccines for all ages, regardless of your insurance status. Appointments for vaccination are available weekly in both our Benzie and Leelanau locations.
We will be happy to bill your insurance. We currently accept most commercial insurances. Call 231-882-4409 ext 3. to discuss.
COVID Vaccine Update
We are have purchased additional Covid-19 vaccine for the 2025-2026 season. Residents can schedule an appointment by calling 231-882-4409 ext 3. or find pharmacy location offering the COVID vaccine: www.vaccines.gov/ or
A nearby retail pharmacy:
MDHHS COVID-19 Vaccine Information
AAP/Healthy Children COVID Vaccine Information
Keep your child up to date on immunizations. Some are required to go to school or daycare.
*Families seeking non-medical immunization waivers for childcare or school entry must call 231-882-4409 ext 3 to schedule an appointment for an in-person meeting with a Health Department Staff Nurse. Waivers are now completed in the MCIR system.
.png)
The Vaccines for Children Program (VFC) provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay. While the vaccine is at no cost, a $23 administrative fee per shot applies to those who qualify. Those unable to pay the administrative fee will not be turned away.
Children through 18 years of age who meet at least one of the following criteria are eligible to receive VFC vaccine:
Children whose health insurance covers the cost of vaccinations are not eligible for VFC vaccines.
Additional information regarding childhood immunization recommendations:
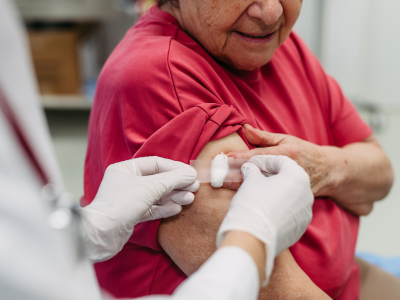
Vaccines aren’t just for kids! As you age, you may become vulnerable to new diseases. Your adult immunization needs depend on your age, lifestyle, medical conditions, travel plans, and vaccination history.
The MI-AVP provides a limited quantity of select vaccines at no cost to adults 19 years and older who might not otherwise be vaccinated because of inability to pay. While the vaccine is at no cost, a $23 administrative fee per shot applies to those who qualify. Those unable to pay the administrative fee will not be turned away.
The following vaccine are available through MI-AVP while supplies last:
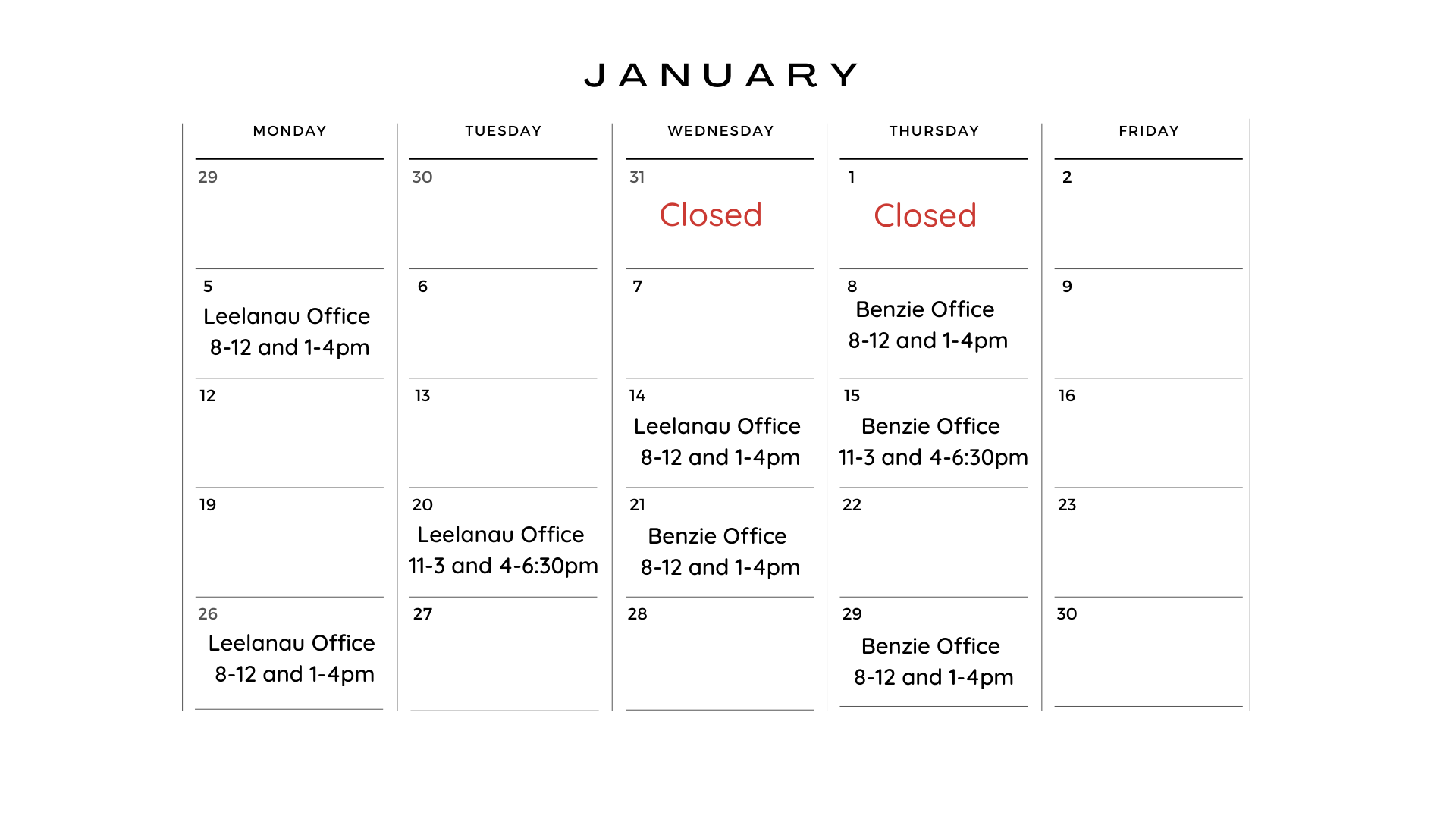
Benzie Office
6051 Frankfort Hwy, Ste 100
Benzonia, MI 49616
Office Hours
Monday-Friday
8am-12pm and 1pm-4:30pm
Phone: (231) 882-4409(231) 882-4409
Fax: (231) 882-2204
Leelanau County
Health Services
7401 E Duck Lake Rd., Ste 100
Lake Leelanau, MI 49653
Office Hours
Monday-Friday
8am-12pm and 1pm-4:30pm
Phone:
(231) 256-0200(231) 256-0200
Fax: (231) 882-0143
Leelanau County
Environmental Services
8527 E. Government Center Dr. Suite LL-007
Suttons Bay, MI 49682
Office Hours
Monday-Friday
8am-12pm and 1pm-4:30pm
Phone:
(231) 256-0201(231) 256-0200
Fax: (231) 256-0225